Introduction
Low-carbohydrate diets are widely used for weight loss, and typically, greater carbohydrate restriction leads to greater weight loss (1, 2, 3, 4). At the most basic level, there are two primary explanations for the ability of low-carbohydrate diets to cause weight loss: either they make use eat fewer calories, or they they make us burn more calories (or both).
So far, we have evidence that low-carbohydrate diets make us eat fewer calories (5, 6), although much of the effect seems to depend on increasing the proportion of protein in the diet rather than restricting carbohydrate per se (7, 8, 9). Yet uncertainty remains over whether or not carbohydrate restriction also increases the metabolic rate, an effect sometimes called a “metabolic advantage”.
Why does this matter? It matters because it tests a hypothesis that has important implications for how we should eat to manage our weight. This is the carbohydrate-insulin hypothesis of obesity. In the version championed by science journalist Gary Taubes, researcher David Ludwig, and others, insulin is a fundamental controller of fat storage due to its direct effects on fat cells, and it controls both calorie intake (hunger) and calorie expenditure (metabolic rate).
According to this idea, insulin is the conductor, and calorie intake and expenditure are simply passengers, of the fattening process. It follows that calorie intake is a red herring, and the only reasonable strategy for weight management is to restrict carbohydrate, and particularly refined carbohydrate.
Unmoved by a substantial body of contradictory evidence (9B), Taubes and others have set out to encourage new studies to test this hypothesis. To do so, they founded an organization called the Nutrition Science Initiative (NuSI), which funds research related to the carbohydrate-insulin hypothesis. I endorsed it (with major reservations) in 2012 because it would provide funding to high-quality scientists and ostensibly would not have the power to tinker with study results (10).
The carbohydrate-insulin hypothesis makes testable predictions that can be used to evaluate it. One of these predictions is that exchanging carbohydrate calories for fat calories, without changing total calorie intake, should increase the metabolic rate and accelerate fat loss. This would be consistent with the notion that calorie intake and expenditure are passengers, not the conductor, of the fattening process. Hot off the presses, the first NuSI-funded study tests this prediction.
Kudos to Taubes and his colleagues for putting their beliefs on the line. But when you do so, you risk that your beliefs will be falsified. Interestingly, certain outcomes the study are consistent with the predictions of the carbohydrate-insulin hypothesis, but the overall picture is devastating to it.
The study
This study was conducted by an impressive group of obesity researchers, including Kevin Hall, Rudy Leibel, Michael Rosenbaum, and Eric Ravussin (11).
The design is quite simple. 17 volunteers with overweight or obesity were kept in a research facility (metabolic ward) for eight weeks. This means they had no opportunities to eat non-study foods. For the first four weeks, they were fed the following diet:
- High-carbohydrate, high-sugar diet (HCD). 50{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} of total calories from carbohydrate (338 g/day), and 25{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} of total calories from sugar. 15{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} protein. 2,739 Calories per day.
For the second four weeks, they were fed the following diet:
- Very-low-carbohydrate, low-sugar ketogenic diet (KD). 5{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} of total calories from carbohydrate (36 g/day), and 2{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} of total calories from sugar. 15{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} protein. 2,738 Calories per day.
The volunteers spent two days a week inside metabolic chambers, where their calorie expenditure was measured. The researchers also used doubly labeled water to measure the volunteers’ average calorie expenditure during the final two weeks of each diet.
Once every two weeks, body composition was measured using dual energy X-ray absorptiometry (DXA), which is a gold-standard method and quite sensitive. They also measured relevant blood markers such as insulin, C-peptide, thyroid hormones, urinary nitrogen, and ketone levels.
It’s important to note that this study had pre-specified primary and secondary outcomes. What this means is that the researchers defined in advance which of the study’s results will be the most important in testing the hypothesis, so that neither they– nor anyone else– have the ability to twist the study’s meaning by cherry-picking data after the fact.
Here are the key outcomes they listed:
- Primary outcomes: changes in total calorie expenditure, changes in sleeping calorie expenditure, and respiratory quotient, as measured by the metabolic chamber (not as measured by doubly labeled water).
- Secondary outcomes: body composition changes, as measured by DXA.
The results
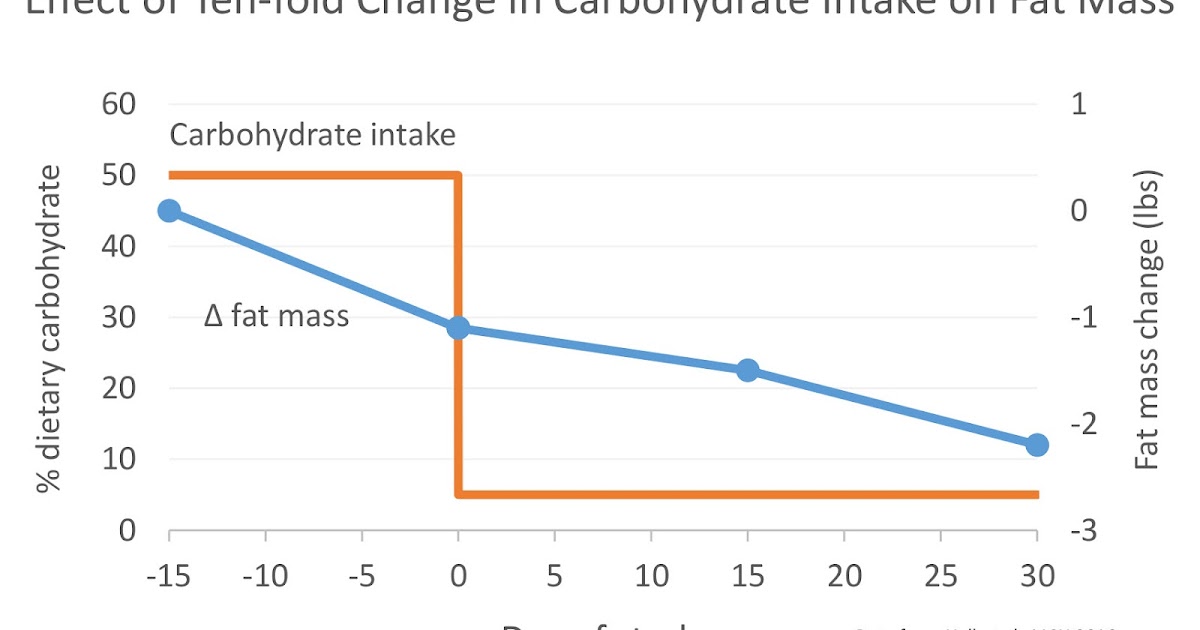
Although the diets were supposed to supply enough calories to keep the volunteers’ weights stable, they slowly lost weight during the HCD, amounting to a loss of 1.1 lbs of body fat over the last two weeks of the diet (body fat loss during the first two weeks was not reported). This suggests that the calories provided on both diets weren’t quite sufficient to maintain weight.
Upon starting the KD, the volunteers rapidly lost weight. This is expected, since low-carbohydrate diets cause a rapid loss of water weight. Yet despite rapid weight loss, their loss of fat mass actually slowed relative to the HCD. Over the first two weeks, they only lost a total of 0.4 lbs of fat. Over the final two weeks, this increased to 0.7 lbs, with a total of 1.1 lbs over the entire one-month KD period. On the KD, the volunteers lost the same amount of body fat in one month that they lost in two weeks on the HCD.
| I prepared this graph from the study data. Feel free to share it. |
Interestingly, the KD did actually increase total calorie expenditure, particularly soon after switching diets. According to the metabolic chamber measurements, volunteers were burning about 100 extra Calories per day for the first ten days or so. Yet this effect waned over time, and by the end of the four-week KD period, total calorie expenditure had dropped approximately back to baseline (~40 extra kcal/day; not statistically significant). Averaged over all metabolic chamber measurements, they burned 57 extra Calories per day on the KD. Changes in sleeping metabolic rate followed a similar trend.
The doubly labeled water measurement indicated a somewhat larger difference in calorie expenditure of 151 kcal/day, favoring the KD. We don’t know how this was changing over time, since this technique gives us one data point that represents the average of two weeks of energy expenditure. It’s likely that if we had more granular data, we would see this gap closing over time, since that’s what the metabolic chamber data suggest.
As expected, insulin secretion declined by 47{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} (as measured by C-peptide) and ketones in urine increased about 11-fold on the KD. Urinary nitrogen excretion increased for the first 11 days of the KD, indicating lean mass breakdown, but this was temporary. Fasting free fatty acid levels increased quite a bit on the KD, but there was no change in overall levels of circulating energy (glucose plus free fatty acids, ketones, glycerol, and triglycerides).
Discussion
This study is interesting because some of its results, if considered superficially, are consistent with the carbohydrate-insulin hypothesis. As predicted by the hypothesis, one of the study’s primary outcomes showed that calorie expenditure increased on the KD. The authors speculate that the increase may have been caused by the metabolic inefficiency of ketogenesis (ketone production) and gluconeogenesis (glucose production), which makes sense*. These are activated by falling insulin, so we can actually say that the increase in calorie expenditure was likely caused by reduced insulin secretion! Although not the supposed effects of insulin on fat cells.
Yet once we take a closer look at the results, they are clearly devastating to the hypothesis. First, the increase in total calorie expenditure was small and transient, and by the end of the one-month period it was no longer measurable using the metabolic chamber, which was a pre-specified primary outcome.
Second and most importantly, the extra calories burned during the KD weren’t coming out of fat tissue! The rate of fat loss actually slowed on the KD, particularly during the first two weeks after the diet transition, where volunteers only lost one third as much fat as they had lost over the previous two weeks of HCD. Over the final two weeks of the KD, fat loss began to rebound, but still only reached two thirds the rate of fat loss of the HCD. In total, the KD caused as much fat loss over one month as the HCD caused over two weeks.
This slowed rate of fat loss on the KD probably happened for two reasons. First, people were burning through their glycogen (carbohydrate) stores in the first few days after switching, as Hall observed in his last metabolic ward study (12). Second, the volunteers were cannibalizing their own lean tissues for protein over the first two weeks of the KD. Because the KD mobilized stored carbohydrate and protein, those extra calories presumably displaced fat calories that would otherwise have been burned. In other words, severe carbohydrate restriction and the resulting drop in insulin cause the body to burn stored carbohydrate and protein at the expense of stored fat. At least initially.
Why did they cannibalize their own lean tissues for protein? It’s probably simply a consequence of the fact that when there isn’t much glucose coming in from the diet, the body starts manufacturing it (gluconeogenesis)– from protein. This increases the body’s protein requirement, and unless the diet is high in protein, the body mines it from lean tissues such as muscle. But in the current study, this effect seemed to be transient, since it tapered off after the first 11 days of the KD. This may explain why the rate of fat loss began to rebound in the last two weeks of the KD period.
The carbohydrate-insulin hypothesis relies on the idea that insulin suppresses the release of fatty acids from fat tissue, reducing overall circulating energy levels and creating a state of “internal starvation” that leads to hunger and sluggishness. If the hypothesis is correct, reducing insulin levels should increase overall circulating levels of energy (glucose plus free fatty acids, ketones, glycerol, and triglycerides) and correct internal starvation. Yet reducing carbohydrate intake from 50{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f} to 5{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f}, and reducing insulin secretion by nearly half, had no effect on overall circulating energy levels. Evidently, the total quantity of circulating energy in the blood is regulated tightly enough that even radical changes in diet have little impact on it.
It’s important to note that in many ways, this study was crafted to maximize the apparent effectiveness of the KD. The KD was very low in carbohydrate (5{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f}), while the HCD was high in carbohydrate (50{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f}) and also very high in sugar (25{e2a5b4b931a58f767699fdd430343808d146204888b8453b8c1ba375f5682a1f}). If you believe the hypothesis that sugar summons Beelzebub to plump up your fat tissue regardless of your calorie intake, the comparison should have been extremely favorable to the KD. Yet the effect on fat mass was the opposite of what this hypothesis predicts.
This study confirms that insulin simply doesn’t work how Taubes, Ludwig, and other insulin-obesity advocates think it does. As the investigators put it, “it is clear that regulation of adipose tissue fat storage is multifaceted and that insulin does not always play a predominant role”. Despite insulin’s well-recognized role in regulating dynamic fatty acid flux in response to meals, circulating insulin levels are not a dominant controller of fat mass. Instead, this study suggests to me that fat tissue plays a more passive role in energy balance: it releases net calories as the body needs them, regardless of what insulin is doing**. Insulin is not the conductor of the fat mass train.
The only remaining explanation for the weight loss produced by low-carbohydrate diets is that they 1) cause a rapid initial loss of water weight, and 2) lead people to eat fewer calories, which gradually depletes fat stores. I do think the second effect is interesting and merits more research. Why do these diets cause a spontaneous reduction in calorie intake, even when people aren’t deliberately trying to restrict calories? Protein is part of the explanation, but I’m not sure it can fully explain what happens when the diet is ketogenic (very low in carbohydrate). I look forward to more research on this.
In many ways, this study was state-of-the-art. It was a true metabolic ward study, so there was no diet cheating. The measurement techniques were gold standard. Yet it did suffer from one puzzling weakness: it lacked a true control group. Therefore, this trial was neither randomized, nor controlled. I don’t know why this decision was made, but it does weaken the result.
This study further reduces my confidence in Ludwig’s finding that a very-low-carbohydrate, high-protein diet increases total calorie expenditure by ~300 Calories per day (13), which Ludwig attributes to the low carbohydrate content of the diet, and subsequent reduction of insulin. This new study suggests that even severe carbohydrate restriction, and a substantial drop in insulin levels, has little impact on the metabolic rate after the first two weeks when protein intake is controlled.
Conclusion
This metabolic ward study suggests that calorie-for-calorie, a very-low-carbohydrate ketogenic diet substantially reduces insulin secretion, transiently increases metabolic rate, and impairs fat loss. As such, it once again falsifies a popular incarnation of the carbohydrate-insulin hypothesis of obesity. Perhaps the fact that this study was funded by NuSI will help the message get through to supporters of the hypothesis.
We can infer that when people eat ketogenic diets outside the lab, they lose fat because they spontaneously reduce their calorie intake. I look forward to more research on why this happens.
* Some energy is lost in the conversion process between protein and glucose, and that extra energy is released from the body as (mostly useless) heat. So for example, if you start with X number of calories of protein, and you convert that into glucose before oxidizing the glucose for energy (primarily ATP), you’ll end up with a smaller quantity of usable energy than if you had just oxidized X calories of glucose directly. Hence the term “inefficiency”. This term carries a negative connotation, but in the context of obesity, metabolic inefficiency can actually be a good thing.
** The reason I say this is that glycogen and protein calories appear to have displaced fat calories in this study, slowing the rate of fat loss in the KD group. In other words, the rate of fatty acid oxidation was determined by the energy demands of the body, not by insulin levels. To qualify my statement, over the longer term fat tissue does play a role in regulating appetite and fat mass, but not by the mechanism proposed by the insulin hypotheis– it does so via its release of leptin.